Gas Hydrates


Methane
250 - 700 trillion cubic feet
more than 50
more than 25
There are 3 methods
Japan
ice-like crystalline minerals that form when low molecular weight gas (such as methane, ethane, or carbon dioxide) combines with water and freezes into a solid under low temperature and moderate pressure conditions. Most gas hydrates are formed from methane (CH4), which has led to the terms “gas hydrate” and “methane hydrate” often being used interchangeably.
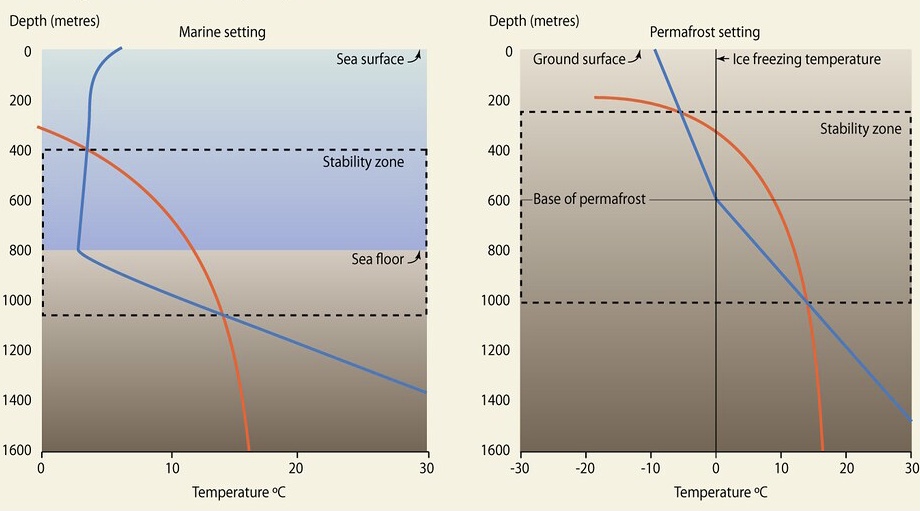
Idealized phase diagrams illustrating where methane hydrate is stable in marine and permafrost settings. Hydrate can exist at depths where the temperature (blue curve) is less than the maximum stability temperature for gas hydrate (orange curve). Pressure and temperature both increase with depth in the Earth.
Gas hydrates present a vast untapped resource of natural gas, with estimates placing hydrate reserves as greater than the known reserves of all oil, natural gas, and coal in the world. Despite this promise, future production volumes are speculative because methane production from hydrate has not been documented beyond small-scale field experiments. Large-scale commercial operations to develop gas-hydrate deposits are at least a decade away.